Microfluidics and Biofluidics: Precision Engineering for Biomedical Innovation
At PhysAI, we harness the power of microfluidics and biofluidics to revolutionize lab-on-chip technologies for biomedical applications. A cornerstone of this field is the development of mixing devices, which enable precise control over fluid dynamics at the microscale. These systems leverage laminar flow regimes, where the Reynolds number (\( Re \)) is typically low, to achieve efficient mixing through diffusion or chaotic advection. For instance, in a serpentine mixer, the Navier-Stokes equations govern fluid behavior:
Using COMSOL Multiphysics, we simulate and optimize these devices to ensure uniform reagent distribution—critical for applications like drug formulation and diagnostic assays in the biomedical sector. These tools allow researchers to iterate designs rapidly, reducing development time and costs while enhancing performance in point-of-care testing platforms.
Cell manipulation, sorting, and separation represent another transformative application of biofluidics. Techniques such as dielectrophoresis (DEP), magnetophoresis, and acoustophoresis exploit external fields to control cell trajectories within microfluidic channels. The force exerted by DEP, for example, depends on the Clausius-Mossotti factor and the electric field gradient:
Coupled with particle tracking methods, these *phoresis mechanisms enable precise sorting of cells—such as isolating circulating tumor cells (CTCs) from blood samples. COMSOL simulations integrate fluid-structure interactions and field gradients, providing insights into optimizing channel geometries for high-throughput diagnostics, a game-changer for personalized medicine and cancer research.
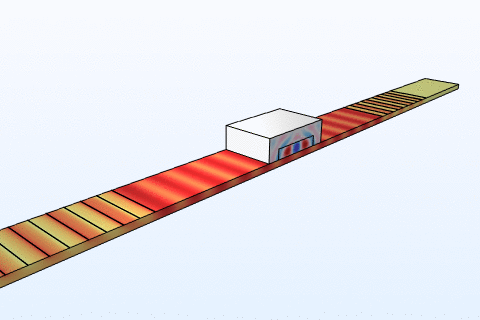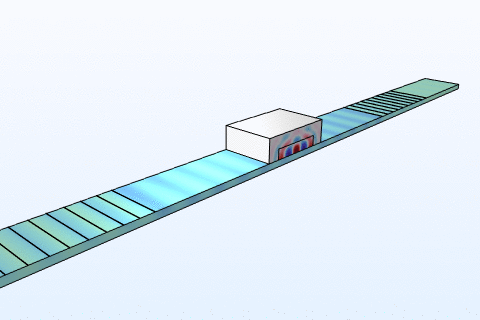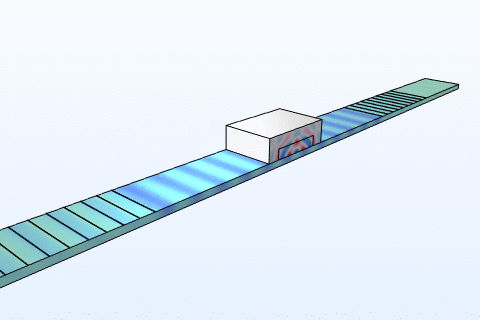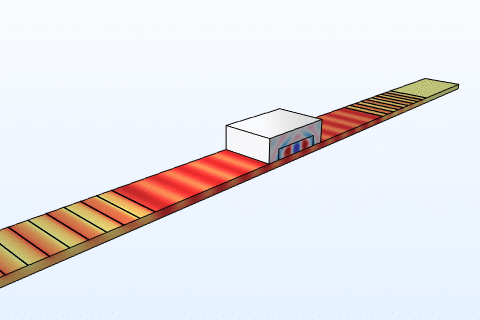
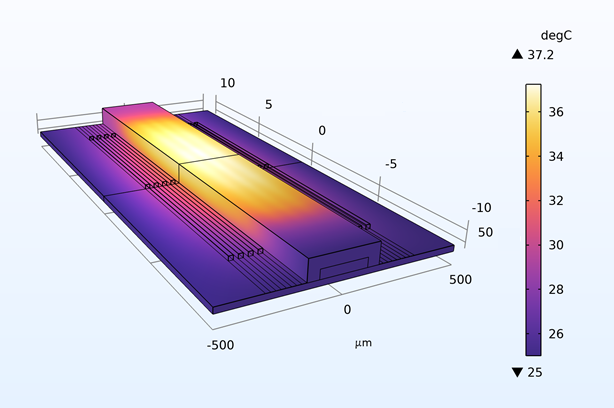
Thermal management is a critical challenge in microfluidic chips, particularly for thermally sensitive bioassays like PCR amplification. Heat transfer in these systems follows the energy equation:
By modeling convection and conduction with COMSOL, we design chips with integrated microheaters and cooling channels to maintain precise temperature profiles. This ensures reliable performance in enzyme reactions and cell viability studies, addressing needs in clinical diagnostics and bioprocessing industries where temperature control directly impacts yield and accuracy.
Liquid filling, handling, and dispensing in microfluidics often involve two-phase flows, such as liquid-gas or liquid-liquid systems. Capillary effects dominate at these scales, governed by the Young-Laplace equation:
Here, \( \mathbf{T}_1 \) and \( \mathbf{T}_2 \) are the total stress tensors for the two fluids, defined as:
These dynamics are pivotal for droplet generation and emulsion production, widely used in drug encapsulation and single-cell analysis. COMSOL’s two-phase flow module allows us to fine-tune surface tension and contact angles, optimizing dispensing precision for applications like automated reagent delivery in pharmaceutical R&D. This capability streamlines workflows, enhancing scalability in high-throughput screening.
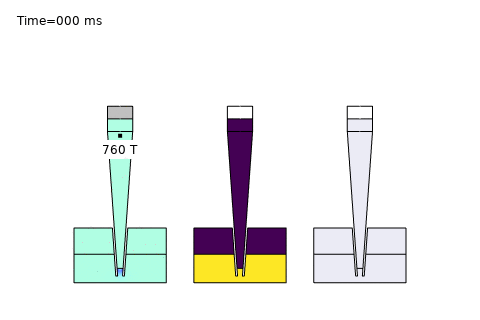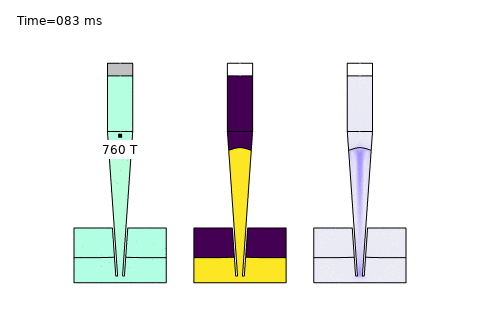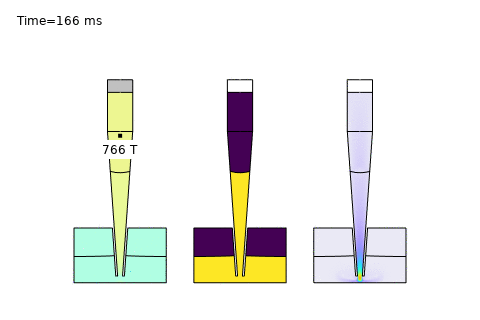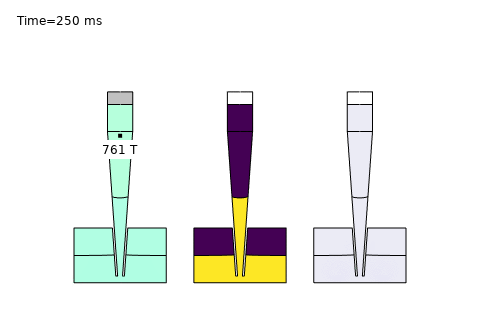
Beyond these core areas, microfluidics is paving the way for cutting-edge applications like single-cell analysis and AI-driven optimization. In single-cell sequencing platforms, precise control of diffusion enables isolation and analysis of individual cells, governed by the Stokes-Einstein relation:
Here, \( D \) is the diffusion coefficient, \( k_B \) is Boltzmann’s constant, \( T \) is temperature, \( \mu \) is viscosity, and \( r \) is the cell radius. COMSOL simulations optimize channel designs for trapping and analyzing single cells, while AI enhances real-time data interpretation—unlocking breakthroughs in precision medicine and genomics. At PhysAI, we’re pushing these frontiers, delivering innovative solutions that empower biomedical pioneers to transform healthcare.


